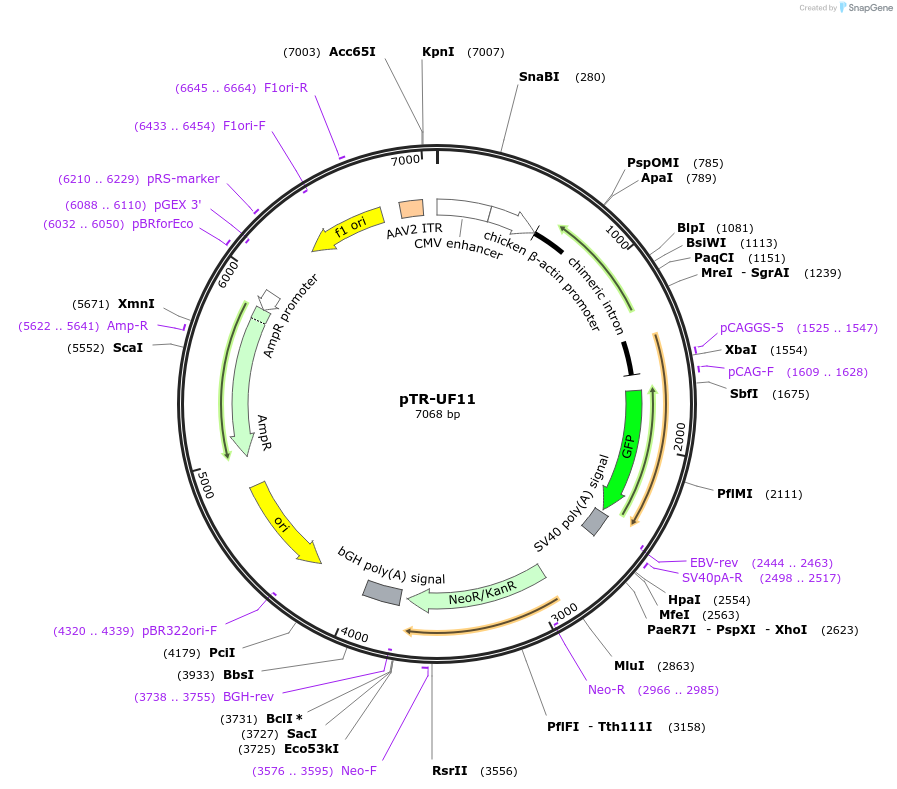
pTR-UF11
(Plasmid
and Viral Vector Preps for
#157970)
-
PurposeAAV vector plasmid containing the Chimeric CMV/Chicken Beta Actin promoter driving 'humanized' GFP
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 157970 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $89 | |
| AAV2 in TMN200 | 157970-AAV.A01.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV2 in PBS | 157970-AAV.A02.T | Virus (20 µL at titer ≥ 5×10¹¹ vg/mL) and Plasmid. | $150 | ||
| AAV2 in BSS | 157970-AAV.A03.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV2 (Y444F) in TMN200 | 157970-AAV.A04.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV2 (Y444F) in BSS | 157970-AAV.A05.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV2 (trpYF) in TMN200 | 157970-AAV.A06.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV2 (trpYF) in BSS | 157970-AAV.A07.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV2(4pMut)dHS in BSS | 157970-AAV.A08.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV6 in TMN200 | 157970-AAV.A09.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV6(dbY-F+T-V) in TMN200 | 157970-AAV.A10.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV44.9 in PBS | 157970-AAV.A11.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
| AAV44.9(E531D) in PBS | 157970-AAV.A12.T | Virus (20 µL at titer ≥ 1×10¹² vg/mL) and Plasmid. | $150 | ||
Don’t see the serotype you want?
Make a packaging request and we'll get back to you.
Please log in to submit a packaging request.
-
SerotypeSelect serotype for details See details about
-
PricingSelect serotype and quantity $ USD for preparation of µL virus + $32 USD for plasmid.
-
How this works
- Place a request for a quantity of 2 (0.2 mL), 10 (1 mL), 25 (2.5 mL), or 50 (5 mL). Our all-inclusive pricing includes DNA production and QC.
- Addgene will quickly confirm that we can produce a high-quality prep for you.
- Track your request and place an order from within your account. Payment information must be added before we can begin processing your order.
- Receive your prep in 6–9 weeks after the MTA is approved by your organization.
- Learn more about our Packaged on Request Service.
Backbone
-
Vector backbonepUC
-
Modifications to backboneContains AAV2 ITRs
-
Vector typeAAV
-
Selectable markersNeomycin (select with G418)
Growth in Bacteria
-
Bacterial Resistance(s)Ampicillin, 100 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)NEB Stable
-
Copy numberHigh Copy
Gene/Insert
-
Gene/Insert name'humanized' e.g. codon optimized GFP
-
Alt namehGFP
-
SpeciesSynthetic
- Promoter chimeric CMV/Chicken Beta actin (CBA)
Cloning Information
- Cloning method Restriction Enzyme
- 5′ cloning site NotI (unknown if destroyed)
- 3′ cloning site NotI (unknown if destroyed)
- 5′ sequencing primer ACAGCTCCTGGGCAACGTGC
- 3′ sequencing primer TGCATTCTAGTTGTGGTTTGTCC
- (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
-
Addgene Notes
-
A portion of this plasmid was derived from a plasmid made byCreated By Sergei Zolotukhin at the University of Florida
-
Article Citing this Plasmid
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
Depositor Comments
This plasmid may be prone to ITR instability. It is recommended to store the plasmid in an E. coli strain such as NEB stable and to verify by SmaI digest. See the Resource Information section for a digest from Addgene's stock.
Information for AAV2 in TMN200 (Catalog # 157970-AAV.A01.T) ( Back to top)
Purpose
Ready-to-use AAV2 in TMN200 particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2 cap gene
- Buffer TMN200 (200mM sodium chloride, 1mM magnesium chloride, 20mM Tris at pH 8.0) supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV2
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
TMN200 is a buffer optimized for maintaining AAV solubility. TMN200 is suitable for in vitro as well as in vivo delivery where the vector is diluted by the physiologic body fluids, such as blood, vitreous, CSF, etc. TMN200 is not suitable as a buffer for vectors delivered directly to the brain parenchyma or subretinal space.Information for AAV2 in PBS (Catalog # 157970-AAV.A02.T) ( Back to top)
Purpose
Ready-to-use AAV2 in PBS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 5×10¹¹ vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2 cap gene
- Buffer PBS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV2
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
PBS is a universal buffer suitable for any application.Information for AAV2 in BSS (Catalog # 157970-AAV.A03.T) ( Back to top)
Purpose
Ready-to-use AAV2 in BSS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2 cap gene
- Buffer BSS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV2
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
BSS is an ophthalmic solution suitable for any application.Information for AAV2 (Y444F) in TMN200 (Catalog # 157970-AAV.A04.T) ( Back to top)
Purpose
Ready-to-use AAV2 (Y444F) in TMN200 particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2(Y444F) cap gene
- Buffer TMN200 (200mM sodium chloride, 1mM magnesium chloride, 20mM Tris at pH 8.0) supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV2(Y444F). The AAV2(Y444F) mutant is derived from AAV2 with the following mutation, Y444F.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
TMN200 is a buffer optimized for maintaining AAV solubility. TMN200 is suitable for in vitro as well as in vivo delivery where the vector is diluted by the physiologic body fluids, such as blood, vitreous, CSF, etc. TMN200 is not suitable as a buffer for vectors delivered directly to the brain parenchyma or subretinal space.Information for AAV2 (Y444F) in BSS (Catalog # 157970-AAV.A05.T) ( Back to top)
Purpose
Ready-to-use AAV2 (Y444F) in BSS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2(Y444F) cap gene
- Buffer BSS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20.
- Serotype AAV2(Y444F). The AAV2(Y444F) mutant is derived from AAV2 with the following mutation, Y444F.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
BSS is an ophthalmic solution suitable for any application.Information for AAV2 (trpYF) in TMN200 (Catalog # 157970-AAV.A06.T) ( Back to top)
Purpose
Ready-to-use AAV2 (trpYF) in TMN200 particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2 (trpYF) cap gene
- Buffer TMN200 (200mM sodium chloride, 1mM magnesium chloride, 20mM Tris at pH 8.0) supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV2 (trpYF). The AAV2 (trpYF) mutant is derived from the AAV2 capsid and contains the following mutations, Y444F, Y500F and Y730F.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
TMN200 is a buffer optimized for maintaining AAV solubility. TMN200 is suitable for in vitro as well as in vivo delivery where the vector is diluted by the physiologic body fluids, such as blood, vitreous, CSF, etc. TMN200 is not suitable as a buffer for vectors delivered directly to the brain parenchyma or subretinal space.Information for AAV2 (trpYF) in BSS (Catalog # 157970-AAV.A07.T) ( Back to top)
Purpose
Ready-to-use AAV2 (trpYF) in BSS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2 (trpYF) cap gene
- Buffer BSS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20.
- Serotype AAV2 (trpYF). The AAV2 (trpYF) mutant is derived from the AAV2 capsid and contains the following mutations, Y444F, Y500F and Y730F.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
BSS is an ophthalmic solution suitable for any application.Information for AAV2(4pMut)dHS in BSS (Catalog # 157970-AAV.A08.T) ( Back to top)
Purpose
Ready-to-use AAV2(4pMut)dHS in BSS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV2(4pMut)dHS cap gene
- Buffer BSS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20.
- Serotype AAV2(4pMut)dHS. The AAV2(4pMut)dHS serotype is derived from AAV2 with the following mutations, Y444F, Y500F, Y730F, T491V, R487G, R585S and R588T.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
BSS is an ophthalmic solution suitable for any application.Information for AAV6 in TMN200 (Catalog # 157970-AAV.A09.T) ( Back to top)
Purpose
Ready-to-use AAV6 in TMN200 particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV6 cap gene
- Buffer TMN200 (200mM sodium chloride, 1mM magnesium chloride, 20mM Tris at pH 8.0) supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV6
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
TMN200 is a buffer optimized for maintaining AAV solubility. TMN200 is suitable for in vitro as well as in vivo delivery where the vector is diluted by the physiologic body fluids, such as blood, vitreous, CSF, etc. TMN200 is not suitable as a buffer for vectors delivered directly to the brain parenchyma or subretinal space.Information for AAV6(dbY-F+T-V) in TMN200 (Catalog # 157970-AAV.A10.T) ( Back to top)
Purpose
Ready-to-use AAV6(dbY-F+T-V) in TMN200 particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV6(dbY-F+T-V) cap gene
- Buffer TMN200 (200mM sodium chloride, 1mM magnesium chloride, 20mM Tris at pH 8.0) supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV6(dbY-F+T-V). The AAV6(dbY-F+T-V) serotype is derived from AAV6 and contains the following mutations, Y705F, Y731F and T492V.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
TMN200 is a buffer optimized for maintaining AAV solubility. TMN200 is suitable for in vitro as well as in vivo delivery where the vector is diluted by the physiologic body fluids, such as blood, vitreous, CSF, etc. TMN200 is not suitable as a buffer for vectors delivered directly to the brain parenchyma or subretinal space.Information for AAV44.9 in PBS (Catalog # 157970-AAV.A11.T) ( Back to top)
Purpose
Ready-to-use AAV44.9 in PBS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV44.9 cap gene
- Buffer PBS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV44.9
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
PBS is a universal buffer suitable for any application.Information for AAV44.9(E531D) in PBS (Catalog # 157970-AAV.A12.T) ( Back to top)
Purpose
Ready-to-use AAV44.9(E531D) in PBS particles produced from pTR-UF11 (#157970). In addition to the viral particles, you will also receive purified pTR-UF11 plasmid DNA.
'Humanized' GFP driven by Chimeric CMV/Chicken Beta Actin promoterDelivery
- Volume 20 µL
- Titer ≥ 1×10¹² vg/mL
- Pricing $118 USD for preparation of 20 µL virus + $32 USD for plasmid.
- Storage Store at -80℃. Thaw just before use and keep on ice.
- Shipment Viral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
- Packaging Plasmids encode adenoviral helper sequences and AAV rep gene, AAV44.9(E531D) cap gene
- Buffer PBS supplemented with 0.001% Poloxamer 188 or 0.014% Tween 20
- Serotype AAV44.9(E531D). The AAV44.9(E531D) serotype is derived from AAV44.9 and contains the following mutation, E531D.
- Purification Iodixanol gradient ultracentrifugation
- Reporter Gene GFP
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert. Biosafety Guide
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Viral Quality Control
- Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC) (see a list of our QC assays). The specific QC assays performed varies for each viral lot. To learn which specific QC assays were performed on your lot, please contact us.
- Titer: the exact titer of your sample will be reported on the tube. The titer you see listed on this page is the guaranteed minimum titer. See how titers are measured.
Visit our viral production page for more information.
Addgene Comments
PBS is a universal buffer suitable for any application.These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
pTR-UF11 was a gift from Sergei Zolotukhin (Addgene plasmid # 157970 ; http://n2t.net/addgene:157970 ; RRID:Addgene_157970) For viral preps, please replace (Addgene plasmid # 157970) in the above sentence with: (Addgene viral prep # 157970-AAV.A01.T), (Addgene viral prep # 157970-AAV.A02.T), (Addgene viral prep # 157970-AAV.A03.T), (Addgene viral prep # 157970-AAV.A04.T), (Addgene viral prep # 157970-AAV.A05.T), (Addgene viral prep # 157970-AAV.A06.T), (Addgene viral prep # 157970-AAV.A07.T), (Addgene viral prep # 157970-AAV.A08.T), (Addgene viral prep # 157970-AAV.A09.T), (Addgene viral prep # 157970-AAV.A10.T), (Addgene viral prep # 157970-AAV.A11.T), or (Addgene viral prep # 157970-AAV.A12.T) -
For your References section:
Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Mol Ther. 2004 Aug;10(2):302-17. doi: 10.1016/j.ymthe.2004.05.024. 10.1016/j.ymthe.2004.05.024 PubMed 15294177