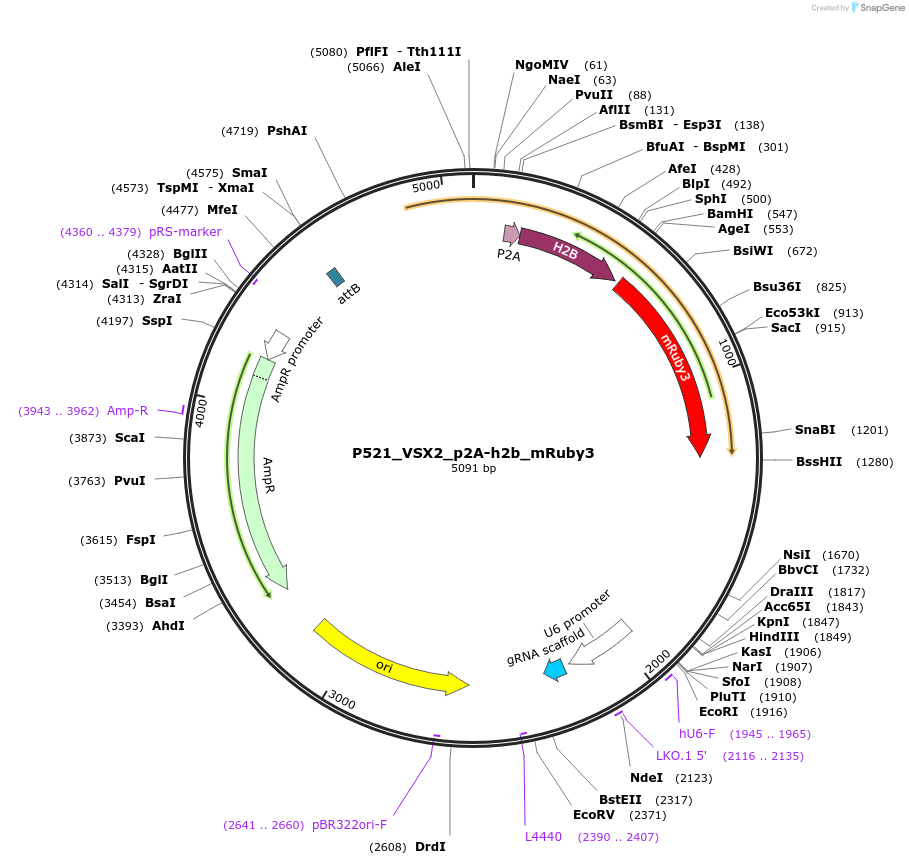
P521_VSX2_p2A-h2b_mRuby3
(Plasmid
#239104)
-
PurposeUsed in CRISPR gene editing to Insert a p2A-h2b_mRuby3 sequence before the stop codon of the endogenous human VSX2 gene
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 239104 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $89 | |
Backbone
-
Vector backbonepUC19
- Total vector size (bp) 5091
-
Modifications to backboneA p2A-h2b-mRuby3 sequence was added in-frame before the stop codon of the endogenous VSX2 gene by Gibson assembly. This plasmid also has a human U6 promoter with a VSX2 targeting guide RNA and scaffold for Cas9 targeting.
-
Vector typeMammalian Expression, CRISPR
Growth in Bacteria
-
Bacterial Resistance(s)Ampicillin, 100 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)NEB Stable
-
Copy numberHigh Copy
Gene/Insert
-
Gene/Insert nameVSX2
-
Alt nameCHX10
-
SpeciesH. sapiens (human)
-
Insert Size (bp)5091
-
Entrez GeneVSX2 (a.k.a. CHX10, HOX10, MCOP2, MCOPCB3, RET1)
- Promoter Endogenous VSX2 promoter
-
Tag
/ Fusion Protein
- -p2A-h2b-mRuby3 (C terminal on insert)
Cloning Information
- Cloning method Gibson Cloning
- 5′ sequencing primer GAGTAGTGCGCGAGCAAAAT
- 3′ sequencing primer GCCTAGGCGGTATCTTCACA
- (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
P521_VSX2_p2A-h2b_mRuby3 was a gift from Karl Wahlin (Addgene plasmid # 239104 ; http://n2t.net/addgene:239104 ; RRID:Addgene_239104) -
For your References section:
Chromatin Accessibility and Transcriptional Differences in Human Stem Cell-Derived Early-Stage Retinal Organoids. Jones MK, Agarwal D, Mazo KW, Chopra M, Jurlina SL, Dash N, Xu Q, Ogata AR, Chow M, Hill AD, Kambli NK, Xu G, Sasik R, Birmingham A, Fisch KM, Weinreb RN, Enke RA, Skowronska-Krawczyk D, Wahlin KJ. Cells. 2022 Oct 28;11(21):3412. doi: 10.3390/cells11213412. 10.3390/cells11213412 PubMed 36359808