Lentiviral Vector Guide
Lentiviruses are a type of retrovirus and belong to the Retroviridae family. Infection with lentiviruses has a long incubation period that eventually leads to serious disease and occurs in many species. Some common types of lentivirus include Human immunodeficiency virus (HIV), bovine immunodeficiency virus (BIV), and feline immunodeficiency virus (FIV).
Retroviruses are partially characterized by their ability to integrate their genomes into hosts in order to continue their lifecycle and replicate. This feature can be harnessed for stable delivery of various genes, mutations, or treatments into cells, and has been widely used in the research community for decades.
Wild-type lentiviruses have been engineered into lentiviral vectors that can be safely used in laboratory settings. These engineered lentiviral vectors have many aspects that make them useful research tools in a variety of cell types and models. Lentiviral vectors can target non-dividing cells, enable stable long-term expression in a host, and exhibit low immunogenicity.
This guide contains many viral vector-specific terms and acronyms, so if you're new to viral vectors or simply need a refresher, we've included a glossary at the end!
Browse lentiviral plasmids available at Addgene.
Lentivirus Versus Gamma-Retrovirus
Lentiviruses and gamma-retroviruses fall under the Retroviridae family. The genome of viruses in this family is made of RNA. This retroviral RNA is reverse transcribed into DNA before being integrated in the genome of a host. Going from RNA to DNA is the reverse of what you would expect in biology — hence the use of “retro”. Lentiviruses have more complex genomes, containing packaging genes and accessory genes specific to each virus type, while retroviruses only contain packaging genes.
From an experimental standpoint, the main difference between lentiviruses and gamma-retroviruses is that lentiviruses are capable of infecting non-dividing and actively dividing cell types, whereas gamma-retroviruses can only infect mitotically active cell types. This means that lentiviruses can infect a greater variety of cell types than retroviruses.
Both lentiviruses and gamma-retroviruses use the same packaging genes. However, they are different viruses and require different isoforms of these packaging components. Therefore, lentivirus may not be efficiently packaged by gamma-retroviral packaging systems, and vice versa.
For more about gamma-retroviruses, see Addgene's gamma-retroviral vector guide.
Lentiviral Vectors
The genome of lentiviruses typically ranges from 8–10 kb, encoded on RNA (Figure 1). This RNA is reverse transcribed into the provirus, the cDNA that will be integrated into a host genome. This single strand of RNA contains packaging genes, regulatory genes, accessory genes, and the long-terminal repeats (LTRs) necessary for integration. Not all of these components are necessary for lentiviral production in the laboratory, and many have been removed or mutated for increased safety.

All retroviruses use the packaging genes gag, pol, and env to create viral particles. Wild-type lentiviruses additionally require the regulatory genes tat and rev, along with virus-specific accessory genes (for example, vif, vpr, vpu, and nef for HIV-1). The LTRs flank all of these genes, and anything in between will be integrated into the host genome. To produce lentiviral vectors in the laboratory, the non-required components have been removed, and the required components have been separated into distinct plasmids for safety. For a summary of all lentiviral plasmid components, see the Lentiviral Plasmid Elements table.
In order to produce lentiviral vectors, you need three (or four, for third generation) plasmids:
- Transfer plasmid — contains transgene, sgRNA, or shRNA of interest flanked by LTRs; 8–10 kb packaging capacity
- Packaging plasmid — contains packaging genes gag and pol, and regulatory genes tat and rev; separated into two plasmids in third generation systems
- Envelope plasmid — contains packaging gene env (usually VSV-G due to wide infectivity)
VSV-G (vesicular stomatitis virus G protein) is the most common envelope gene in all systems, due to a wide range of infectivity for different cell types (known as tropism). Lentiviral vectors have gone through improvements over the years for efficiency and safety. These improvements have been divided into different generations.
Lentiviral Generations
First-generation
First-generation lentiviral plasmids are largely defunct due to their lack of safety improvements and the potential for replication-competent lentivirus production — that is, creation of viral particles that could infect cells and further replicate on their own. While the viral components are still separated on different plasmids, the viral genome remains largely intact.
First-generation plasmids include (Figure 2):
- Transfer plasmid — contains transgene and wild-type LTRs
- Packaging plasmid — contains entire viral genome (packaging, regulatory, and accessory genes), only envelope removed
- Envelope plasmid — contains env

Second-generation
Second-generation lentiviral plasmids greatly improved the safety of lentiviral vector production by removing the accessory genes. Second-generation plasmids include tat, as the 5’ LTR is used as a promoter on the transfer plasmid, and this requires Tat for activation.
Second-generation plasmids include (Figure 3):
- Transfer plasmid — contains transgene and wild-type LTRs
- Packaging plasmid — contains gag, pol, tat, and rev
- Envelope plasmid — contains env
All second-generation lentiviral transfer plasmids must be used with a second-generation packaging system because transgene expression from the LTR is Tat-dependent.

Third-generation
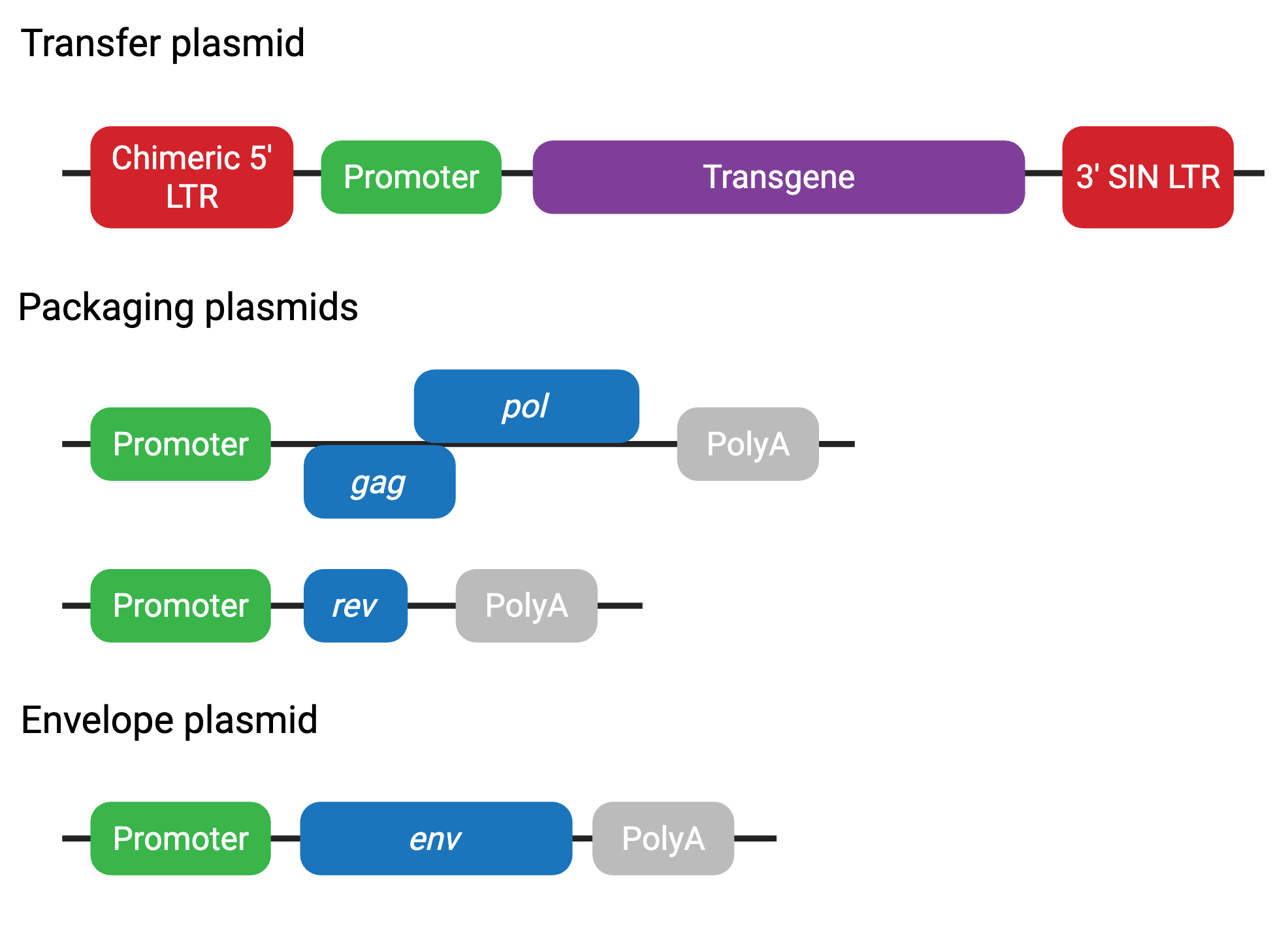
The third-generation system further improves on the safety of the second generation in a few key ways. First, the packaging system is split into two plasmids. Although safer, this system can be more cumbersome to use and result in lower viral titers due to the additional plasmid. Second, tat is not required in the third-generation system. Instead, the transfer plasmid contains a chimeric 5’ LTR fused to a heterologous promoter (often CMV or RSV), eliminating the need for transactivation by Tat.
Most third-generation transfer plasmids also contain a deletion in the 3’ LTR to make them self-inactivating (SIN). This deletion is transferred to the 5’ LTR after one round of reverse transcription, inhibiting transcription of the full-length virus after it is incorporated into a host genome. This is often found in second-generation transfer plasmids as well.
Third-generation plasmids include (Figure 4):
- Transfer plasmid — contains transgene and LTRs (chimeric 5’ LTR)
- Packaging plasmid 1 — contains gag and pol
- Packaging plasmid 2 — contains rev
- Envelope plasmid — contains env
Third-generation transfer plasmids can be packaged by either second- or third-generation packaging plasmids. For a summarized comparison of second- and third-generation systems, please refer to Table 1.
| Feature | Second-generation | Third-generation |
|---|---|---|
| Transfer Plasmid | Can be packaged ONLY by a second-generation packaging system that includes the tat gene | Can be packaged by both second- and third-generation packaging systems |
| Packaging Plasmid | One plasmid encoding gag, pol, tat, and rev | Two plasmids, one encoding gag/pol and another encoding rev |
| Envelope Plasmid | Interchangeable; usually encodes for VSV-G | Interchangeable; usually encodes for VSV-G |
| Safety | Safe; replication incompetent by using three separate plasmids encoding the necessary viral genes | Safer; replication incompetent by using four plasmids instead of three and eliminates the requirement for Tat. Always includes deletion in the 3' LTR for self-inactivation |
| LTR Viral Promoter | Wildtype | Hybrid promoter; 5' LTR is partially deleted and fused to a heterologous enhancer/promoter such as CMV or RSV |
Table 1. Summary of key differences between second- and third-generation lentiviral plasmids.
Lentiviral Vector Production
Cloning
Cloning your transgene, gRNA, or shRNA of interest into the transfer plasmid can be done with most standard cloning methods, including restriction enzyme, Gibson Assembly, or Gateway. Some transfer plasmids may have limited restriction sites or may only be compatible with certain cloning methods (such as a Gateway destination vector), so be sure to confirm your chosen plasmid is compatible with cloning methods available in your lab.
When cloning your plasmids, be sure to use recombination-deficient bacteria strains, such as NEB Stable cells. These strains reduce the frequency of homologous recombination of unstable regions, like the LTRs found in lentiviral plasmids. This will ensure that the repeats will be maintained and often results in a greater yield of DNA. However, if the plasmid contains a Gateway cassette containing the ccdB gene, a ccdB-resistant strain is necessary.
For more information on cloning and working with plasmids, visit Addgene’s Molecular Biology Reference.
Production
After cloning, you will need to produce the lentiviral vectors from your plasmids (Figure 5). This process requires a production cell line, typically human embryonic kidney 293T (HEK293T) cells. Standard HEK293T cells are sufficient, but there are some HEK293T cell lines that have been specifically engineered for lentiviral production.
All lentiviral plasmids (three or four) are transfected into HEK293T cells using your preferred transfection method. The HEK293T cells will produce the viral vectors and release them into the supernatant. After an initial media change, the supernatant containing the viral vectors is removed and stored or centrifuged to concentrate. You can use crude or concentrated viral particles to transduce the cells of interest, either with or without determining the viral titer.
This process of viral production followed by transduction is necessary to properly utilize lentiviral vectors. Some lentiviral plasmids (mostly third-generation) can be used in transient transfections, but expression will be greatly limited due to the LTRs. Generally, lentiviral plasmids cannot be used in direct transfections for transient expression of a transgene.
For more detailed protocols, see Addgene’s viral vector protocols.

Viral Vector Integration
Genome-wide studies of viral integration have shown that lentiviruses most often integrate into transcriptionally active regions, regions recently involved in translocation events, and other “fragile” genomic locations, and that this preference is conserved across target species. Although chromatin availability facilitates integration, it does not explain the lentiviral preference for transcribed genes. Studies comparing the lentivirus HIV and the gamma-retrovirus MMLV indicate that the viral integrase plays a role in shaping integration site preferences. A major cellular determinant is LEDGF (also known as PSIP1 or p75), a lentiviral tethering gene that helps facilitate integration.
Despite knowing the general preference for integration, it is still random and difficult to predict. In therapeutic applications, like chimeric antigen receptor T cell (CAR-T) therapy, the location of integration can greatly affect outcomes and potential side effects. Efforts to more accurately predict and understand integration sites have led to the development of multiple genome-wide analysis pipelines to assess viral integration (see Kim et al. (2021), Timms et al. (2018), and Wells et al. (2020) for examples).
Pseudotyping
Tropism dictates which types of host cells the lentiviral vector will infect. This can be altered by changing the envelope gene, a process called pseudotyping. VSV-G is by far the most common envelope gene, as it has a wide range of infectivity across many cell types and enhances the stability of the viral particles. However, VSV-G is toxic under long-term, constitutive expression. In order to make stable cell lines, VSV-G is often put under an inducible promoter. Depending on the cell type, there are other envelope gene options, including:
- Lymphocytic Choriomeningitis Virus (LCMV) – less toxic and inflammatory; targets wide range of cell types
- Ebola glycoprotein — transduce airway epithelium and cardiomyocytes in utero
- Rabies glycoprotein — transduce neural progenitor and stem cells
Chimeric envelope genes have also gained popularity to further enhance both infectivity and specificity. Pseudotyped lentiviral vectors are common in clinical applications, like gene therapy, as you can more specifically dictate the target cell population and reduce cellular toxicity.
Common Uses of Lentiviral Vectors
Due to their integration and long-term expression of a transgene, lentiviral vectors are useful research and clinical tools.
Stable Cell Lines
Lentiviral vectors can be used to make stable cell lines due to their integration into a host’s genome. Many lentiviral vectors have selectable markers, such as the puromycin resistance gene, conferring antibiotic resistance to infected host cells. When these antibiotics are added to the growth medium of the host cells, they kill off any cells that have not incorporated the lentiviral genome and the cells that survive can be expanded to create stable cell lines. The cell lines will have the lentiviral genome and the genetic information encoded by that genome.
Antibiotic resistance is not the only type of selectable marker. Fluorescent reporters, such as GFP, are another common selection marker. Instead of selection by an antibiotic, fluorescence-activated cell sorting (FACS) is used to sort cells expressing GFP and later expanded into a cell line. More information on generating cell lines with lentiviral vectors can be found in Addgene’s stable cell line protocol.
Browse Addgene's collection of in-stock lentiviral preps.
CRISPR Genomic Screens
Genome-wide screens are an efficient way to determine what genes may be involved in different biological processes by surveying a pool of genetic mutants for various phenotypes. These screens involve hundreds to even thousands of target genes and require gRNAs for each target. Lentiviral vectors are a common way of delivering these gRNAs, due to their stable expression and wide tropism. Addgene has an extensive collection of lentiviral CRISPR libraries, including knockout, inhibition/activation, and prime and base editing.
Browse Addgene's collection of CRISPR libraries.
Cellular Barcoding
Much like genomic screens, lentiviral vectors are a preferred delivery method when conducting cellular barcoding experiments. Lentiviral vectors allow for integration of the barcodes, which can then be inherited by daughter cells. Inheritance of the barcodes makes lentiviral libraries popular for lineage tracing experiments and monitoring the dynamics of heterogeneous tumors.
Browse Addgene's collection of barcoding libraries.
Mouse Modeling
Since the development of safer lentiviral vectors, they have been seen as an effective method for in vivo gene delivery. Lentiviral vectors are commonly used to create transgenic mice with heritable mutations or transgene expression. Popular uses include Cre-lox mice, knockouts using CRISPR or shRNA, and overexpressing of oncogenes and other disease-associated genes.
Gene Therapy
Due to their relative safety and ability to transduce non-dividing cells, lentiviral vectors are also popular in clinical settings for the delivery of gene therapies. Lentiviral vectors are particularly effective for stem cell therapies, which rely on slowly proliferating cell populations that can be difficult to transduce by other means. A concern with gene therapies is the possibility of transducing healthy tissues not associated with a certain disease and causing unwanted side effects. This concern can be partially mitigated through the use of tissue-specific promoters and envelope genes, and/or with site-specific injections. Despite the possibility of nonspecific transduction, lentiviral gene therapies have been shown to be relatively safe and effective.
Viral Vector Safety
The two main safety concerns surrounding the use of lentiviral vectors are:
- The potential for generation of replication-competent lentiviral vectors
- The potential for oncogenesis
The potential for generation of replication-competent lentiviral vectors is very low thanks to the design of second- and third-generation lentiviral vectors. Second- and third-generation vectors separate transfer, envelope, and packaging components of the virus between different plasmids. The transfer plasmid encodes the gene of interest and contains the sequences that will be incorporated into the host cell genome but cannot produce functional viral particles without the genes encoded in the envelope and packaging plasmids. Unless multiple recombination events occur between the packaging, envelope, and transfer plasmids, and the resulting construct is packaged into a viral particle, it is not possible for viral vectors to replicate after the initial infection.
Third-generation systems are considered safer than second-generation systems because the packaging components have been divided into two plasmids, resulting in a four-plasmid system in total. In addition, third-generation systems do not require the HIV protein Tat to activate the promoter on the transfer plasmid.
Many of the lentiviral transfer plasmids that have been deposited with Addgene are self-inactivating (SIN) plasmids. These plasmids have a deletion in the 3' LTR of the viral genome that is transferred into the 5' LTR after one round of reverse transcription. After incorporation into a host cell, this deletion prevents further transcription of the full-length virus.
The potential for oncogenesis is largely based on whether or not the specific insert contained within the lentiviral transfer plasmid is an oncogene. Additional consideration for oncogenes should be made on a case-by-case basis.
As with most experiments, infection risks occur with contact to mucous membranes or broken skin. Needle sticks and ripped gloves are common points of entry. Biosafety should always be considered with respect to the precise nature of experiments being performed. Your biosafety office can provide more information on your institution's best practices with regard to lentiviral-related research.
See Addgene’s Biosafety Resource Guide for more information (including tropism and viral genome percentage) and resources on viral safety.
Resources and References
Resources on addgene.org
Resources on Addgene's Blog
Addgene Protocols
References
Cronin, J., Zhang, X., & Reiser, J. (2005). Altering the Tropism of Lentiviral Vectors through Pseudotyping. Current Gene Therapy, 5(4), 387–398. https://doi.org/10.2174/1566523054546224 PMID: 16101513
Dull, T., Zufferey, R., Kelly, M., Mandel, R. J., Nguyen, M., Trono, D., & Naldini, L. (1998). A Third-Generation Lentivirus Vector with a Conditional Packaging System. Journal of Virology, 72(11), 8463–8471. https://doi.org/10.1128/jvi.72.11.8463-8471.1998 PMID: 9765382
Durand, S., & Cimarelli, A. (2011). The inside out of lentiviral vectors. Viruses, 3(2), 132–159. https://doi.org/10.3390/v3020132 PMID: 22049307
Ghaleh, H. E. G., Bolandian, M., Dorostkar, R., Jafari, A., & Pour, M. F. (2020). Concise review on optimized methods in production and transduction of lentiviral vectors in order to facilitate immunotherapy and gene therapy. Biomedicine & Pharmacotherapy, 128, 110276. https://doi.org/10.1016/j.biopha.2020.110276 PMID: 32502836
Johnson, N. M., Alvarado, A. F., Moffatt, T. N., Edavettal, J. M., Swaminathan, T. A., & Braun, S. E. (2021). HIV-based lentiviral vectors: Origin and sequence differences. Molecular Therapy — Methods & Clinical Development, 21, 451–465. https://doi.org/10.1016/j.omtm.2021.03.018 PMID: 33981779
Kim, H. S., Hwang, G., Lee, H. K., Bae, T., Park, S., Kim, Y. J., Lee, S., Park, J., Bae, S., & Hur, J. K. (2021). CReVIS-Seq: A highly accurate and multiplexable method for genome-wide mapping of lentiviral integration sites. Molecular Therapy — Methods & Clinical Development, 20, 792–800. https://doi.org/10.1016/j.omtm.2020.10.012 PMID: 33768124
Marshall, H. M., Ronen, K., Berry, C., Llano, M., Sutherland, H., Saenz, D., Bickmore, W., Poeschla, E., & Bushman, F. D. (2007). Role of PSIP1/LEDGF/p75 in Lentiviral Infectivity and Integration Targeting. PLoS ONE, 2(12), e1340. https://doi.org/10.1371/journal.pone.0001340 PMID: 18092005
Merten, O., Hebben, M., & Bovolenta, C. (2016). Production of lentiviral vectors. Molecular Therapy — Methods & Clinical Development, 3, 16017. https://doi.org/10.1038/mtm.2016.17 PMID: 27110581
Naldini, L., Blömer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M., & Trono, D. (1996). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science, 272(5259), 263–267. https://doi.org/10.1126/science.272.5259.263 PMID: 8602510
Schambach, A., Zychlinski, D., Ehrnstroem, B., & Baum, C. (2013). Biosafety features of lentiviral vectors. Human Gene Therapy, 24(2), 132–142. https://doi.org/10.1089/hum.2012.229 PMID: 23311447
Shalem, O., Sanjana, N. E., Hartenian, E., Shi, X., Scott, D. A., Mikkelsen, T. S., Heckl, D., Ebert, B. L., Root, D. E., Doench, J. G., & Zhang, F. (2013). Genome-Scale CRISPR-CAS9 knockout screening in human cells. Science, 343(6166), 84–87. https://doi.org/10.1126/science.1247005 PMID: 24336571
Shao, L., Shi, R., Zhao, Y., Liu, H., Lu, A., Ma, J., Cai, Y., Fuksenko, T., Pelayo, A., Shah, N. N., Kochenderfer, J. N., Norberg, S. M., Hinrichs, C., Highfill, S. L., Somerville, R. P., Panch, S. R., Jin, P., & Stroncek, D. F. (2022). Genome-wide profiling of retroviral DNA integration and its effect on clinical pre-infusion CAR T-cell products. Journal of Translational Medicine, 20(1). https://doi.org/10.1186/s12967-022-03729-5 PMID: 36348415
Stewart, S. A., Dykxhoorn, D. M., Palliser, D., Mizuno, H., Yu, E. Y., An, D. S., Sabatini, D. M., Chen, I. S., Hahn, W. C., Sharp, P. A., Weinberg, R. A., & Novina, C. D. (2003). Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA, 9(4), 493–501. https://doi.org/10.1261/rna.2192803 PMID: 12649500
Timms, R. T., Tchasovnikarova, I. A., & Lehner, P. J. (2018). Differential viral accessibility (DIVA) identifies alterations in chromatin architecture through large-scale mapping of lentiviral integration sites. Nature Protocols, 14(1), 153–170. https://doi.org/10.1038/s41596-018-0087-5 PMID: 30518911
Wells, D. W., Guo, S., Shao, W., Bale, M. J., Coffin, J. M., Hughes, S. H., & Wu, X. (2020). An analytical pipeline for identifying and mapping the integration sites of HIV and other retroviruses. BMC Genomics, 21(1). https://doi.org/10.1186/s12864-020-6647-4 PMID: 32151239
Zufferey, R., Dull, T., Mandel, R. J., Bukovsky, A., Quiroz, D., Naldini, L., & Trono, D. (1998). Self-Inactivating Lentivirus Vector for safe and efficient in vivo gene delivery. Journal of Virology, 72(12), 9873–9880. https://doi.org/10.1128/jvi.72.12.9873-9880.1998 PMID: 9811723
Zufferey, R., Nagy, D., Mandel, R. J., Naldini, L., & Trono, D. (1997). Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nature Biotechnology, 15(9), 871–875. https://doi.org/10.1038/nbt0997-871 PMID: 9306402
Lentiviral Plasmid Elements
| Plasmid Type | Element | Delivery relative to transgene | Purpose |
|---|---|---|---|
| Transfer plasmid | LTR | in cis | Long terminal repeat; comprised of a U3-R-U5 structure and are found on each side of the provirus. The U3 (unique 3’) contains sequences necessary for activation of viral genomic RNA transcription. R is the repeat region. |
| U3 | in cis | Unique 3’; contains sequences necessary for activation of viral genomic RNA transcription. Removal of this region in the 3’ LTR in third-generation plasmids creates self-inactivating viral vectors. | |
| R | in cis | Repeat region where Tat binds. | |
| TAR | in cis | Trans-activating response element; found in the R region and acts as the binding site for Tat. | |
| U5 | in cis | Unique 5'; in third-generation plasmids, this region is often removed in 5’ LTRs and replaced with a heterologous promoter (CMV or RSV). | |
| 5' LTR | in cis | Acts as an RNA pol II promoter; the transcript begins, by definition, at the beginning of R, is capped, and proceeds through U5 and the rest of the provirus. Third-generation plasmids use a hybrid 5' LTR with a constitutive promoter such as CMV or RSV. | |
| 3' LTR | in cis | Terminates transcription started by 5' LTR by the addition of a polyA tract just after the R sequence. | |
| WPRE | in cis | Woodchuck hepatitis virus post‐transcriptional regulatory element; stimulates the expression of transgenes via increased nuclear export. | |
| RRE | in cis | Rev Response Element; the sequence to which the Rev protein binds. | |
| Psi (Ѱ) | in cis | RNA packaging signal; recognized by nucleocapsid proteins and essential for efficient viral packaging. | |
| cPPT | in cis | Central polypurine tract; recognition site for proviral DNA synthesis. Increases transduction efficiency and transgene expression. | |
| Packaging plasmid | gag | in trans | Precursor structural protein of the lentiviral particle containing matrix, capsid, and nucleocapsid components. |
| pol | in trans | Precursor protein containing reverse transcriptase and integrase components. | |
| rev | in trans | Binds to the Rev Response Element (RRE) within unspliced and partially spliced transcripts to facilitate nuclear export. Provided by a separate plasmid from gag/pol in third-generation packaging plasmids. | |
| tat | in trans | Trans-activator; binds TAR to activate transcription from the LTR promoter. Only in first- and second-generation lentiviral plasmids. | |
| Envelope plasmid | env | in trans | The viral envelope gene; typically vesicular stomatitis virus G glycoprotein (VSV-G), an envelope protein with broad tropism used to pseudotype most lentiviral vectors. |
Glossary
| Term | Definition |
|---|---|
| Immunogenicity | The ability of a molecule or substance to induce an immune response in the body. |
| in cis | In the context of viral vector production, in cis refers to genetic elements located in the same plasmid as the gene of interest. |
| in trans | In the context of viral vector production, in trans refers to genetic elements provided outside of the plasmid containing the gene of interest (i.e., other plasmids, packaging cell line). |
| Infection | The natural process of entry and multiplication of a pathogen, like viruses or bacteria, within a host organism, leading to disease. |
| Lentivirus | A retrovirus from the Retroviridae family. Characterized by long incubation and the eventual cause of serious diseases. |
| Provirus | The genetic material (cDNA for retroviruses) that will be integrated into a host cell’s genome. |
| Pseudotyping | The production of viral vectors (or viruses) using viral envelope proteins from different species. Used to alter infectivity and tropism. |
| Replication-competent | The ability of a virus (or viral vector) to replicate and reproduce within host cells. In viral vectors, can occur from a spontaneous crossover event between the transfer plasmid and genes provided in trans. |
| Transduction | The process of artificially introducing foreign DNA into eukaryotic cells using viral methods. |
| Transformation | The process where bacteria take up DNA from their environment. |
| Tropism | The specific types of cells and tissues that a virus can infect and replicate in. |
| Viral vector | The modified form of a wild-type virus used to deliver genetic material into different host cells. Engineered for safety and efficiency. |
Credits
- Contributing Authors
- Written and reviewed by the Scientific Curation team at Addgene.
- Media Credits
- Figures created with BioRender (Link opens in a new window)
- Last Updated
- Content last reviewed on 9 June 2025.


