pUS250
(Plasmid
#198322)
-
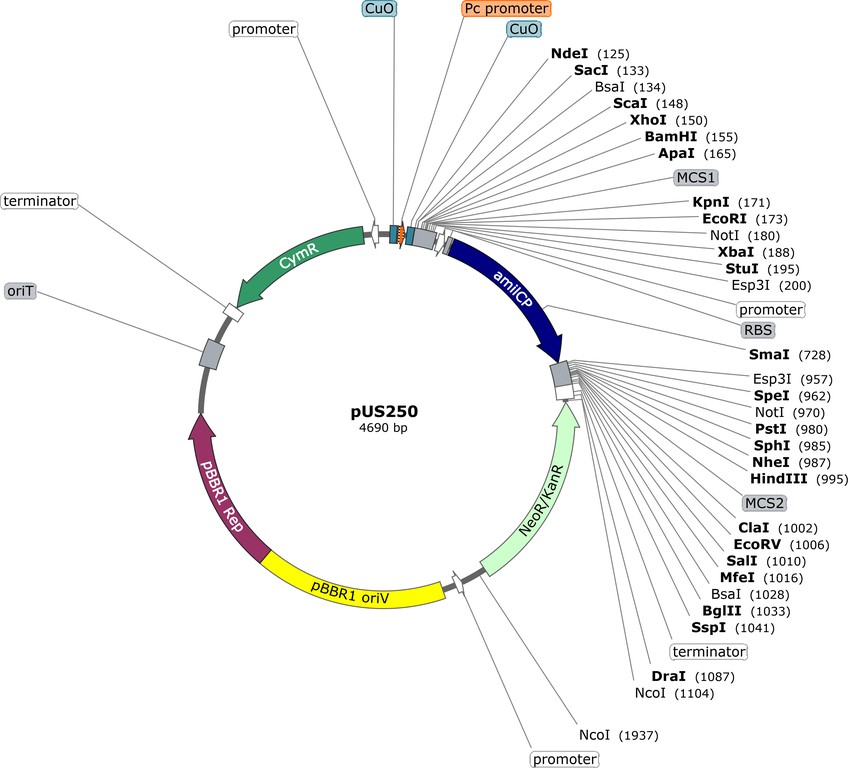
Purpose(Empty Backbone) Cumate-inducible gene expression in diverse gram negative bacteria. Features also include mobilisation via oriT, a gigantic multiple cloning site, and blue/white screening via amilCP chromoprotein
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 198322 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $85 * | |
* Login to view industry pricing.
Backbone
-
Vector backbonepUS250
-
Backbone manufacturerColeman
- Backbone size (bp) 4690
-
Vector typeBacterial Expression, Synthetic Biology
- Promoter cumate-inducible
Growth in Bacteria
-
Bacterial Resistance(s)Kanamycin, 50 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)DH5alpha
-
Copy numberLow Copy
Cloning Information
- Cloning method Restriction Enzyme
- 5′ sequencing primer GTTCGGTTCGTAAACTGTAATGC
- 3′ sequencing primer CCCGTGATATTGCTGAAGAG (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
-
A portion of this plasmid was derived from a plasmid made byn/a
-
Article Citing this Plasmid
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
Trademarks:
- Zeocin® is an InvivoGen trademark.
Depositor Comments
CLONING: The multiple cloning site (MCS) in pUS250 functions differently to a typical MCS. You need to choose one unique restriction site on the left side of the amilCP marker gene (e.g. EcoRI), and one unique restriction site on the right side (e.g. PstI) in order for the blue/white selection and the cumate-inducible expression features to work properly. Cutting the plasmid in this way excises the amilCP gene, replacing it with your gene of interest, and changing the phenotype from blue to white. The vector is also compatible with GoldenGate cloning (using either BsaI or Esp3I) and BioBrick cloning (iGEM). In the case of GoldenGate cloning, you don't need to use two different enzymes, you just use one or the other, since each enzyme has two sites, one on each side of amilCP, and each yields a different overhang.
HOST RANGE and EXPRESSION: we have shown that pUS250 can be used for cumate-inducible gene expression in E.coli, Pseudomonas putida, and Rhizobium leguminosarum. It is likely that the plasmid will also be useful in other gram negatives (Proteobacteria) since the pBBR replicon has a very broad host range. The plasmid can be transferred by conjugation from E.coli strains such as S17 or SM10 into other species due to the oriT sequence. For the E.coli and Rhizobium, 100 uM cumate is sufficient for expression. For Pseudomonas, this needs to be increased to 10 mM (we think there is an efflux pump that pumps cumate back out of these cells). The cumate should be added after autoclaving. We make a 0.5 M cumate stock solution by mixing equal parts of 1M aqueous Tris base with 1M cumic acid in ethanol. Cumate is an excellent inducer since it is both cheap and non-toxic to both bacteria and people. You can even use cumin (the spice) for induction of gene expression in this plasmid! (there is enough cumate in the cumin).
STABILITY and COPY NUMBER: We estimate that the vector has a copy number of 5-10 in E.coli, so it is certainly a low copy vector. Best to make large-scale plasmid preps (e.g. 50 ml culture) rather than small-scale, in order to ensure you get enough plasmid DNA to work with. The plasmid is quite stable but we have seen white mutants appear occasionally which have deletions in amilCP.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
pUS250 was a gift from Nicholas Coleman (Addgene plasmid # 198322 ; http://n2t.net/addgene:198322 ; RRID:Addgene_198322)