Chemogenetics Guide
Chemogenetic tools are actuators for specific cellular pathways, receptors, or ion channels targeted to specific cell populations (most often neurons) that can be turned on or off by the application of a small molecule ligand. The ideal chemogenetic tools are unresponsive to native ligands and are engineered to respond to small molecules that do not affect endogenous signaling, therefore allowing precise control over the cell population they are targeted to.
Read this guide to learn more, or explore Addgene's Chemogenetics Plasmid Collection.

Early Chemogenetic Receptors: RASSLs
The first chemogenetic receptors were based on G-protein coupled receptors (GPCRs). The largest class of cell surface receptors, GPCRs are seven-pass transmembrane proteins that bind a specific ligand. Ligand binding then activates G-proteins to modulate downstream signaling. GPCRs have been shown to be involved in a wide variety of biological processes, including initiating signaling pathways in inflammation and neurotransmission. This, and the fact that GPCR ligands can have high potency, which is potentially important for dosing in animal studies, made them attractive targets for development into chemogenetic tools.
Studies of GPCRs led to development of mutated receptors that while unresponsive to endogenous ligands, could be activated by synthetic ligands. Specifically, Receptors Activated Solely by Synthetic Ligands (RASSLs) based on κ-opiod receptors (Coward et al. 1998). These receptors were activated by the synthetic ligand spiradoline, however, spiradoline exhibited off-target effects in vivo and the receptors exhibited high levels of constitutive activity, making them less than ideal.
DREADDs
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), like RASSLs, are based on engineered G-protein coupled receptors, but unlike RASSLs, show insensitivity to endogenous ligands, have low constitutive activity, and their activating ligands have few, if any off-target effects. They are the most widely employed type of chemogenetic receptors. There are several different types of DREADDs that can be broadly classified by the signaling protein that the receptors couple to:
Gq-DREADDs
Gq-DREADDs signal through the Gαq/11 G-protein and activate neuronal firing through stimulating phospholipase C, which releases intracellular calcium stores. There are currently three Gq DREADDs based on human muscarinic receptors: hM1Dq, hM3Dq, and hM5Dq, with hM3Dq being the most widely used.
Gi-DREADDs
Gi-DREADDs signal through the Gαi/o G-protein and inhibit neuronal signaling by inhibiting adenylate cyclase and downstream cAMP production. There are currently two Gi DREADDs based on human muscarinic receptors, hM2Di and hM4Di, and a Gi DREADD based on the human κ-opoid receptor, termed KORD (or KORDi).
Gs-DREADDs
Gs-DREADDs signal through the Gαs G-protein and activate neuronal signaling by increasing intracellular cAMP concentrations. There is currently one Gs-DREADD, rM3D, that was created by replacing the corresponding intracellular region of a turkey erythrocyte β-adrenergic receptor with a rat M3 muscarinic receptor. This DREADD was shown to have a small amount of constitutive activity, and is not widely used.
There is also a DREADD that couples to β-arrestin to activate noncanonical GPCR signaling independent of G proteins. This DREADD, termed Rq(R165L), is based on the human M3 muscarinic receptor and has not been used in vivo.

DREADD Ligands
Muscarinic-receptor based DREADDs were engineered to respond to nM concentrations of clozapine N‐oxide (CNO). CNO is a metabolite of the antipsychotic clozapine and seems to be pharmacologically inert in mice and rats. However, CNO is back-metabolized to clozapine and other clozapine metabolites and these can have off-target effects. More recent studies have shown that CNO does not cross the blood-brain barrier in rats, and the utility of DREADDs in experiments in this species may be entirely due to back-metabolism of CNO to clozapine, demonstrating the need for alternative DREADD ligands.
Compound 21, deschloroclozapine (DCZ), perlapine, and olanzapine have all been explored as alternative ligands for muscarinic-receptor based DREADDs. Compound 21 has similar potency as CNO, while DCZ is more potent than either CNO or Compound 21. Both Compound 21 and DCZ seem to have minimal off-target activity, and do not seem to have the same back-metabolism issues as CNO and are attractive alternatives for experiments. Perlapine has been previously used in human populations in Japan, making it an attractive option for translational studies, however, it has off-target effects. The atypical antipsychotic drug olanzapine has also been shown to be an agonist against hM4Di, and is especially attractive for use in translational studies because it is FDA and EMA approved.
One pitfall of a suite of muscarinic-based DREADDs that all respond to the same ligands was that bidirectional experiments (i.e. using both activating and inhibiting DREADDs in the same experimental setup) were not possible. To overcome this limitation, the inhibitory KORD DREADD was developed to respond to a different ligand, Salvinorin B (SALB). Scientists have shown that both KORD and hM3Dq can be used in the same organism to allow bidirectional control of neuronal activity.
Table 1: Descriptions of DREADDs and their activity in neurons
| DREADD | Receptor | Effector | Ligand | Effect | Outcome (in neurons) | Reference |
|---|---|---|---|---|---|---|
| Rq(R165L) | Human M3 muscarinic | Arrestin-2/-3 | CNO* | Increase Arrestin translocation | Arrestin signalling | Nakajima & Wess, 2012 |
| hM3Dq hM1Dq hM5Dq | Human M3 muscarinic | Gαq | CNO* | Increase Ca2+ | Neuronal burst firing | Armbruster et al., 2007 |
| rM3D | Rat M3 muscarinic & turkey β1-adrenergic | Gαs | CNO* | Increase cAMP | Neuronal burst firing | Guettier et al., 2009 |
| hM4Di hM2Di | Human M4 muscarinic | Gαi | CNO* | Decrease cAMP | Neuronal inhibition | Armbruster et al., 2007 |
| KORD | Human κ-opioid | Gαi | SALB | Decrease cAMP | Neuronal inhibition | Vardy et al., 2015 |
* For a discussion of alternative DREADD ligands, please see the text.
PSAMs
Unlike DREADDs, which manipulate neuronal activity indirectly through GPCR signaling, another class of chemogenetic receptors that confer more direct control of neurons through manipulation of ion channels arePharmacologically Selective Actuator Modules (PSAMs, pronounced SAMs). PSAMs are engineered α7 nicotinic acetylcholine receptor (nAChR) domains that respond to specific small molecules termed Pharmacologically Selective Effector Molecules (PSEMs). PSAM domains were first named for the mutations that allowed them to respond to their cognate PSEM. For example, PSAMQ79G,Q139G is activated by PSEM22S, while PSAML141F,Y115F is activated by PSEM89S. Later, in an effort to develop a PSAM that responded to a clinically approved drug, the PSAM domain was engineered to respond specifically to the anti-smoking drug varenicline. This PSAM carries mutations at L131G, Q139L, and Y217F and is termed PSAM4 (or PSAM4). Additional varenicline analogs, termed ultrapotent PSEMs (or uPSEMs), have also been developed as agonists for PSAM4. A given PSAM is coupled with an ion pore domain (IPD) to form ligand-gated ion channels (LGICs) that can either excite or inhibit neuronal signaling. PSAM-based LGICs can be classified by the ion pore domain they are coupled to:
PSAM-Gly
PSAM-Gly LGICs pair a PSAM domain with a Glycine-receptor (GlyR) chloride-selective IPD. Binding of the cognate PSEM allows for the influx of Cl- ions, and inhibits neuronal activity.
PSAM-5HT3
PSAM-5HT3 LGICs pair a PSAM domain with a 5-HT3 serotonin receptor sodium- or potassium-selective IPD. Binding of the cognate PSEM allows for influx of Na+ and/or K+ ions, and activates neuronal activity.

Table 2: Descriptions of PSAMs and their activity in neurons
| PSAM | Ion Pore Domain | Ligand(s) | Effect | Outcome (in neurons) | Reference |
|---|---|---|---|---|---|
| PSAM4 | Gly | Varenicline, uPSEM 792, uPSEM 817 | Cl- influx | Neuronal inhibition | Magnus et al., 2019 |
| PSAM4 | 5HT3 | Varenicline, uPSEM 792, uPSEM 817 | Na+, K+ influx | Neuronal activation | Magnus et al., 2019 |
| PSAMQ79G,Q139G | Gly | PSEM22S | Cl- influx | Neuronal inhibition | Magnus et al., 2011 |
| PSAMQ79G,Q139G | 5HT3 | PSEM22S | Na+, K+ influx | Neuronal activation | Magnus et al., 2011 |
| PSAML141F,Y115F | Gly | PSEM89S | Cl- influx | Neuronal inhibition | Magnus et al., 2011 |
| PSAML141F,Y115F | 5HT3 | PSEM89S | Na+, K+ influx | Neuronal activation | Magnus et al., 2011 |
LMOs
Luminopsins (LMOs), or luminescent opsins, are opto-chemogenetic fusion proteins of a light-emitting luciferase and a light-sensing optogenetic element. When the luciferase substrate (luciferin) is added, the luciferase enzyme generates light that activates the opsin. The opsin may excite or inhibit the neurons expressing the LMO, depending on its biophysical properties. While LMOs provide chemogenetic access to optogenetic tools, they also retain the ability to be activated by standard optogenetics workflows (such as laser or LED illumination), making LMOs a versatile option. Coupling brighter bioluminescent modules, such as luciferase-fluorescent protein fusions, to more-sensitive opsins has led to improved LMOs with increased efficiencies. Depending on the type of luciferase used, the luciferin substrate may be the native coelenterazine (CTZ) or chemical variations thereof (hCTZ), or furimazine (Fz) or chemical variations thereof (FFz, CFz).
Learn more about optogenetic systems in our Optogenetics Guide.

Table 3: Descriptions of LMOs and their activity in neurons
| LMO | Luciferase | Opsin | Ligand | Effect | Outcome (in neurons) | Reference |
|---|---|---|---|---|---|---|
| LMO3 | sbGLuc | VChR1 | CTZ | Na+, K+ influx | Neuronal activation | Berglund et al., 2016 |
| LMO7 | mNeonGreen-eKL9h | VChR1 | CFz | Na+, K+ influx | Neuronal activation | Björefeldt et al., 2024 |
| LMO11 | GeNL_SS | ChRmine | CFz | Na+, K+ influx | Neuronal activation | Slaviero et al., 2024 |
| iLMO: RLuc-NpHR | Venus-Rluc8 | NpHR | hCTZ | Cl- influx | Neuronal inhibition | Tung et al., 2015 |
| iLMO: GeNL_SS- hGtACR1 | GeNL_SS | hGtACR1 | CFz | Cl- influx | Neuronal inhibition | Ikefuama et al., 2025 |
Plan Your Chemogenetics Experiment
When planning your chemogenetics experiment, here are some things to consider:
Activation or Inhibition?
Do you want to turn ON or turn OFF the neurons in your experiment? Depending on your answer, you'd pick a chemogenetic activator or inhibitor, respectively.
DREADDs or PSAMs or LMOs?
DREADDs are monomeric proteins, while PSAMs have more than one domain, both of which need to be expressed. However, neuronal control through PSAMs and LMOs is direct, while neuronal control of DREADDs is indirect. DREADD ligands affect signaling for up to 8 hours after delivery, while PSAMs and LMOs have an effect for only 0.5–1 hours after delivery.
Chemogenetic Ligand
The chemogenetic actuator chosen and the context of the experiment will determine the chemogenetic ligand used. For example, a study in rats using an hM4Di receptor might consider an alternative DREADD ligand such as DCZ versus CNO.
Delivery
Chemogenetic actuators are delivered in vivo through viral injection or use of genetically engineered mouse models. For viral injection, Adeno-associated virus (AAV) is a widely-used tool for achieving in vivo expression of chemogenetic receptors. Check out Addgene's collection of ready-to-use AAV preparations of chemogenetic plasmids. Additionally, genetically engineered animal models expressing hM3Dq, hM4Di, and rM3D DREADDs and LMOs have been developed and are commercially available.
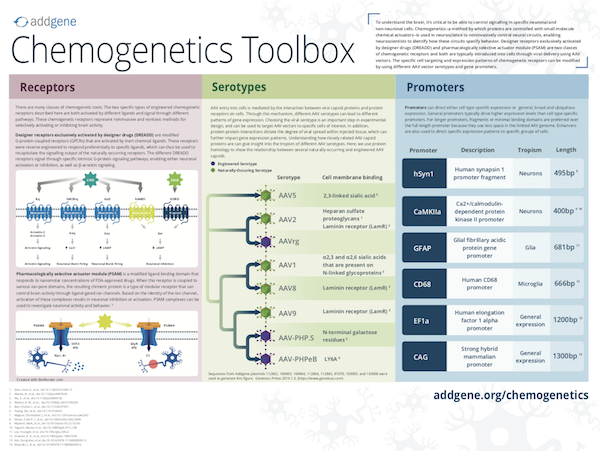
Table 4: Common promoters in chemogenetics plasmids
| Promoter | Cell Specificity |
|---|---|
| hSyn1, CaMKIIa | Neurons |
| GFAP | Glia |
| CD68 | Microglia |
| Dlx | Interneurons |
| EF1a, CAG | General expression |
Targeted Expression
Depending on the experiment, an AAV-encoded chemogenetic plasmid may need to be targeted to specific tissues, cell types or even subcellular regions of a neuron. Cell-specific expression of AAV-delivered constructs can be controlled with cell-type specific promoters. Table 4 lists some common promoters found in chemogenetic receptor constructs and the cell types in which they drive expression.
Different AAV serotypes can be used to target AAV vectors to specific cells of interest. In addition, different AAV serotypes have varying degrees of viral spread within injected tissue, and this must be considered. For tissue specificity of AAV serotypes, see our AAV guide.
FLEx Vectors are also used to achieve cell-specific expression of AAV-encoded chemogenetic receptors. FLEx vectors block expression in the absence of Cre and are especially useful in AAV experiments where the virus can spread around the injection site and potentially express the chemogenetic receptor in unwanted cell types. Generating a FLEx switch to control expression of a chemogenetic reporter ensures that the reporter remains silent until a cell or tissue-specific Cre is provided.
Download our chemogenetics poster:
References and Further Reading
Atasoy, D., & Sternson, S. M. (2018). Chemogenetic Tools for Causal Cellular and Neuronal Biology. Physiol Rev, 98(1), 391–418. https://doi.org/10.1152/physrev.00009.2017 PMID: 29351511
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S., Roth, B.L. (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA, 104(12), 5163–5168. https://doi.org/10.1073/pnas.0700293104 PMID: 17360345
Berglund, K., Clissold, K., Li, H. E., Wen, L., Park, S. Y., Gleixner, J., Klein, M. E., Lu, D., Barter, J. W., Rossi, M. A., Augustine, G. J., Yin, H. H., & Hochgeschwender, U. (2016). Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation. Proc Natl Acad Sci USA, 113(3), E358–E367. https://doi.org/10.1073/pnas.1510899113 PMID: 26733686
Björefeldt, A., Murphy, J., Crespo, E. L., Lambert, G. G., Prakash, M., Ikefuama, E. C., Friedman, N., Brown, T. M., Lipscombe, D., Moore, C. I., Hochgeschwender, U., & Shaner, N. C. (2024). Efficient opto- and chemogenetic control in a single molecule driven by FRET-modified bioluminescence. Neurophotonics 11(2), 021005. https://doi.org/10.1117/1.NPh.11.2.021005 PMID: 38450294
Coward, P., Wada, H. G., Falk, M. S., Chan, S. D., Meng, F., Akil, H., & Conklin, B. R. (1998). Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci USA, 95(1), 352–357. https://doi.org/10.1073/pnas.95.1.352 PMID: 9419379
Davies, M. A., Compton-Toth, B. A., Hufeisen, S. J., Meltzer, H. Y., & Roth, B. L. (2004). The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: is M1 agonism a pre-requisite for mimicking clozapine's actions? Psychopharmacology, 178(4), 451–460. https://doi.org/10.1007/s00213-004-2017-1 PMID: 15765260
Farrell, M. S., Pei, Y., Wan, Y., Yadav, P. N., Daigle, T. L., Urban, D. J., Lee, H. M., Sciaky, N., Simmons, A., Nonneman, R. J., Huang, X. P., Hufeisen, S. J., Guettier, J. M., Moy, S. S., Wess, J., Caron, M. G., Calakos, N., & Roth, B. L. (2013). A Gαs DREADD Mouse for Selective Modulation of cAMP Production in Striatopallidal Neurons. Neuropsychopharmacol, 38(5), 854–862. https://doi.org/10.1038/npp.2012.251 PMID: 23303063
Gomez, J. L., Bonaventura, J., Lesniak, W., Mathews, W. B., Sysa-Shah, P., Rodriguez, L. A., Ellis, R. J., Richie, C. T., Harvey, B. K., Dannals, R. F., Pomper, M. G., Bonci, A., & Michaelides, M. (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357(6350), 503–507. https://doi.org/10.1126/science.aan2475 PMID: 28774929
Guettier, J. M., Gautam, D., Scarselli, M., Ruiz de Azua, I., Li, J. H., Rosemond, E., Ma, X., Gonzalez, F. J., Armbruster, B. N., Lu, H., Roth, B. L., & Wess, J. (2009). A chemical-genetic approach to study G protein regulation of β cell function in vivo. Proc Natl Acad Sci USA, 106(45), 19197–19202. https://doi.org/10.1073/pnas.0906593106 PMID: 19858481
Ikefuama, E. C., Slaviero, A. N., Silvagnoli, A. D., Crespo, E. L., Schalau, R., Gott, M., Tree, M. O., Dunbar, G. L., Rossignol, J., & Hochgeschwender, U. (2025). Presymptomatic targeted circuit manipulation for ameliorating Huntington's disease pathogenesis. iScience, 28(3), 112022. https://doi.org/10.1016/j.isci.2025.112022. PMID: 40092615
Magnus, C. J., Lee, P. H., Atasoy, D., Su, H. H., Looger, L. L., & Sternson, S. M. (2011). Chemical and genetic engineering of selective ion channel-ligand interactions. Science, 333(6047), 1292–1296. https://doi.org/10.1126/science.1206606 PMID: 21885782
Magnus CJ, Lee PH, Bonaventura J, Zemla R, Gomez JL, Ramirez MH, Hu X, Galvan A, Basu J, Michaelides M, Sternson SM (2019). Ultrapotent chemogenetics for research and potential clinical applications. Science, 364(6436), eaav5282. https://doi.org/10.1126/science.aav5282 PMID: 30872534
Nagai, Y., Miyakawa, N., Takuwa, H., Hori, Y., Oyama, K., Ji, B., Takahashi, M., Huang, X. P., Slocum, S. T., DiBerto, J. F., Xiong, Y., Urushihata, T., Hirabayashi, T., Fujimoto, A., Mimura, K., English, J. G., Liu, J., Inoue, K. I., Kumata, K., Seki, C., … Minamimoto, T. (2020). Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci, 23(9), 1157–1167. https://doi.org/10.1038/s41593-020-0661-3 PMID: 32632286
Nakajima, K., & Wess, J. (2012). Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol, 82(4), 575–582. https://doi.org/10.1124/mol.112.080358 PMID: 22821234
Roth, B.L. (2016). DREADDs for Neuroscientists. Neuron, 89(4), 683–694. https://doi.org/10.1016/j.neuron.2016.01.040 PMID: 26889809
Slaviero, A. N., Gorantla, N., Simkins, J., Crespo, E. L., Ikefuama, E. C., Tree, M. O., Prakash, M., Björefeldt, A., Barnett, L. M., Lambert, G. G., Lipscombe, D., Moore, C. I., Shaner, N. C., & Hochgeschwender, U. (2024). Engineering luminopsins with improved coupling efficiencies. Neurophotonics, 11(2), 024208. https://doi.org/10.1117/1.NPh.11.2.024208 PMID: 38559366
Sternson, S.M., Roth, B.L. (2014). Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci, 37, 387–407. https://doi.org/10.1146/annurev-neuro-071013-014048 PMID: 25002280
Strader, C. D., Gaffney, T., Sugg, E. E., Candelore, M. R., Keys, R., Patchett, A. A., & Dixon, R. A. (1991). Allele-specific activation of genetically engineered receptors. J Biol Chem, 266(1), 5–8. https://doi.org/10.1016/S0021-9258(18)52392-9 PMID: 1670767
Tung, J. K., Gutekunst, C. A., & Gross, R. E. (2015). Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci Rep, 5, 14366. https://doi.org/10.1038/srep14366 PMID: 26399324
Vardy, E., Robinson, J. E., Li, C., Olsen, R. H. J., DiBerto, J. F., Giguere, P. M., Sassano, F. M., Huang, X. P., Zhu, H., Urban, D. J., White, K. L., Rittiner, J. E., Crowley, N. A., Pleil, K. E., Mazzone, C. M., Mosier, P. D., Song, J., Kash, T. L., Malanga, C. J., Krashes, M. J., … Roth, B. L. (2015). A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron, 86(4), 936–946. https://doi.org/10.1016/j.neuron.2015.03.065 PMID: 25937170
Weston, M., Kaserer, T., Wu, A., Mouravlev, A., Carpenter, J. C., Snowball, A., Knauss, S., von Schimmelmann, M., During, M. J., Lignani, G., Schorge, S., Young, D., Kullmann, D. M., & Lieb, A. (2019). Olanzapine: A potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Sci Adv, 5(4), eaaw1567. https://doi.org/10.1126/sciadv.aaw1567 PMID: 31001591